zinc stearate reaction
Raising temperature from room temperature RT to 45C or 90C allowed the reaction to proceed faster. It works as an antacid with polyolefins contributing to colour stability and preventing corrosion.
Zinc Stearate C36h70o4zn Pubchem
Zinc stearate facilitates dispersion by being very soluble in the apolar region of polyolefins.

. Water and zinc stearate were formed. At 600 C no acid soap was detected. Table 2 shows the effect of zinc stearate on mold release at 28 PHR SR633 in ethylene vinyl acetate copolymer Elvax 240.
Zinc stearate has good thermal stability initial stage stainability is small it has good light stability and it has. Incompatible with oxidizing agents dilute acids. The make up is.
Zinc Stearate is a material that is easily soluble in water ultra-fine and with good dispersion compatibility. Conveying liquid stearic acid by a pump allowing the liquid stearic acid to go through a flow meter for metering and then enter a zinc stearate reaction vessel stirring and heating. Adding zinc oxide four times and reacting at a temperature of 160DEG C under a.
TABLE I FATTY ACIDS LENGTH LINEAR MELTPOINTC. At 600 C no acid soap was detected. Zinc stearate has different ratios of palmitic and stearic acids.
Reaction must be removed from the metallic stearate by filtration and washing. At the same time it also has the function of vulcanization activator and softener in rubber. These salts are produced from a reaction with stearic acid fatty acid and a metal oxide.
Toluene helped zinc stearate to turn into the corresponding methyl esters by making zinc stearate partly soluble in a raised temperature as well as making the reaction product soluble. 1 Dinuclear type bridging bidentate zincstearate complex labelled as the intermediate I where X is hydroxyl group water andor rubber segment. Caprate 050 Laurate 25 Palmitate 28-57 Stearate 28-68 Myristic 3-5 Pentadecanoic up to 050 Margaric Acid up to 2 Oleic acid up to 050 Arachidic and Behenic acid 30-40 Lauric Acid up to 050 Typical.
Zinc stearate is an activator for vulcanization because zinc has a beneficial effect on the reaction of sulphur with polyolefin. The invention discloses a zinc stearate production technology. Water and zinc stearate were formed.
EVA optimum results were achieved at 5 phr zinc stearate. ZINC STEARATE is non-flammable but combustible. It is a white powder and is insoluble in water.
The invention belongs to the technical field of chemical engineering and particularly relates to high-quality zinc stearate prepared from glyceryl tristearate. 2 zinc oxide and a catalyst are added stepwise. Aluminium and magnesium stearates are used as foam inhibitors for various suspensions.
From 280 to 375 C zinc stearate was present in the reaction products of the acid with both zinc metal and zinc oxide. - As a heat stabilizer and internal lubricant in the manufacture of PVC products. This chemical is an example of metal soaps and is insoluble in water and polar solvents such as alcohol.
For zinc and zinc oxide the first reaction zone began at about 160 C and extended to 280290 C. Zinc stearate occurs by the reaction of interaction of stearic acid with calcium hydroxide with heating and vigorous stirring. Mold release at this concentration was good and above this zinc stearate level properties appear to diminish as shown in Figure 2.
Mainly used as lubricant and release agent for styrene resin phenolic resin and amino resin. From 280 to 375 C zinc stearate was present in the reaction products of the acid with both zinc metal and zinc oxide. Zinc stearate is produced from various reactions including the reaction of zinc oxide and stearic acid which is shown in the following.
EVA Mold Release. Belongs to the Following Reactive Group s Esters Sulfate Esters Phosphate Esters Thiophosphate Esters and Borate Esters. The technology comprises the following steps.
Emits acrid smoke and fumes of ZnO when heated to decomposition Hazardous Chemicals Desk Reference p. A preparation technology of zinc stearate comprises the following steps that 1 glyceryl tristearate and water are hydrolyzed under the action of a catalyst and an antioxidant. Figure 2 Table 2.
The newly observed zincstearate complex acts as an enzyme to accelerate the sulfur cross-linking reaction of rubber. For zinc and zinc oxide the first reaction zone began at about 160 C and extended to 280290 C. Calcium stearate magnesium stearate and zinc stearate have stabilising and processing aid effects in a wide range of thermoplastics.
It is valued by manufacturers because of the delicate feeling it gives to the finished products good heat resistance excellent transparency yellowing resistance quick-drying and increased sandability. Considering the molar masses of each chemical substance in the reaction gmol -1 Zn 814 gmol -1 stearic acid 2845 gmol -1 and water 18 gmol -1 and the respective stoichiometric. For example the zinc stearate will melt during molding and be absorbed into the compound without leaving discoloration or defects on the surface of the final molded rubber part.
Finally the resulting wet cake must. Zinc stearate is an organic substance with a chemical formula of C36H70O4Zn.

Reaction Between Zno And Stearic Acid Forming Zinc Stearate And The Download Scientific Diagram
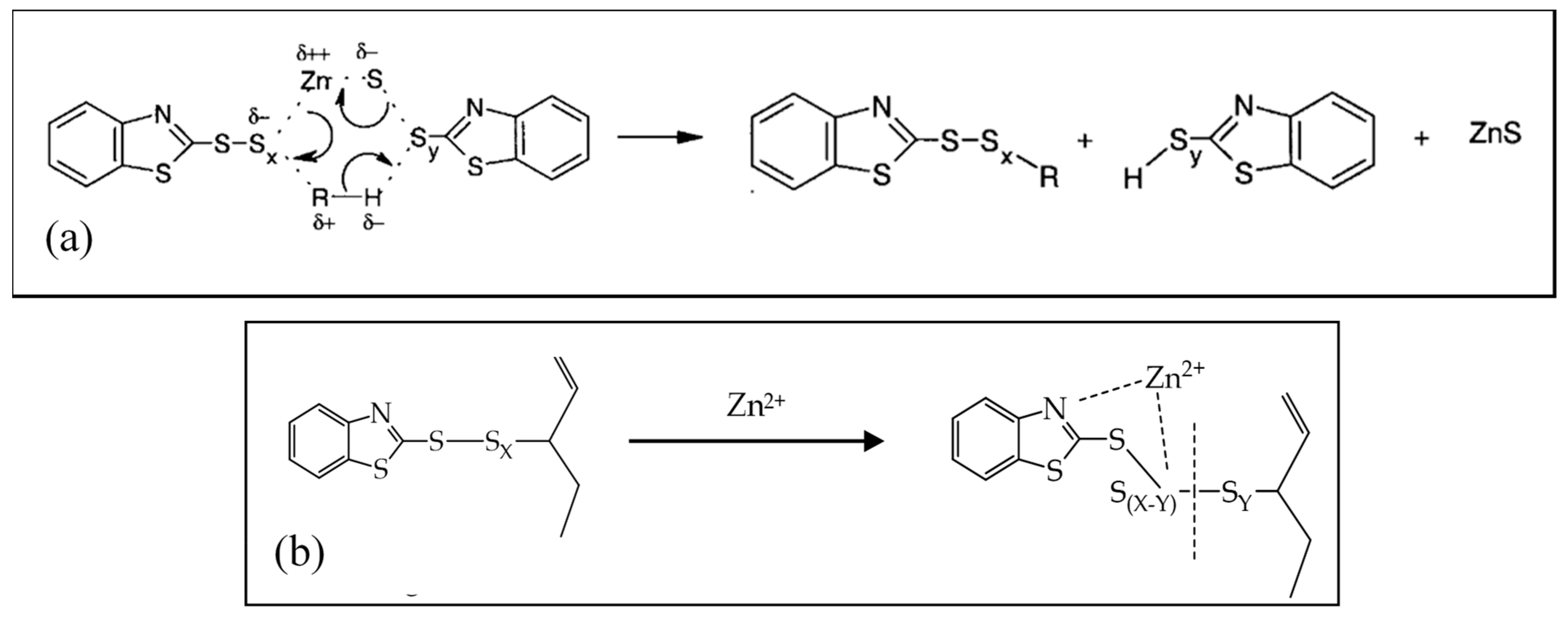
Catalysts Free Full Text Zinc Based Curing Activators New Trends For Reducing Zinc Content In Rubber Vulcanization Process Html

Reaction Between Zno And Stearic Acid Forming Zinc Stearate And The Download Scientific Diagram

Novel Approach For Rapid Oil Water Separation Through Superhydrophobic Superoleophilic Zinc Stearate Coated Polyurethane Sponges Sciencedirect

Measured Characteristics Of The Prepared Zinc Stearate Download Table
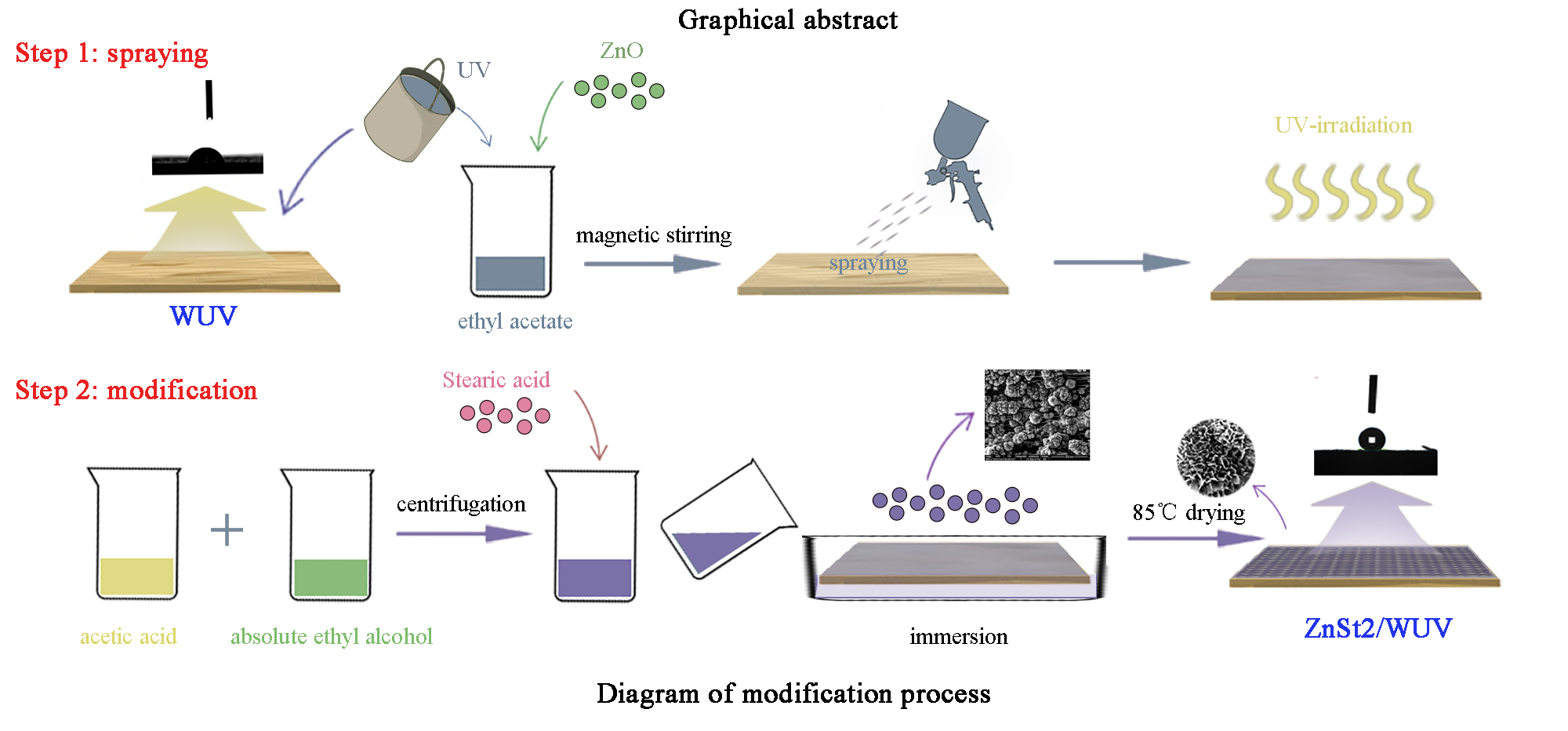
Polymers Free Full Text Preparation And Characterization Of Waterborne Uv Lacquer Product Modified By Zinc Oxide With Flower Shape Html

Effect Of Synthesized Zinc Stearate On The Properties Of Natural Rubber Vulcanizates In The Absence And Presence Of Some Fillers Sciencedirect
Reaction Between Zno And Stearic Acid Forming Zinc Stearate And The Download Scientific Diagram

Zinc Stearate Formation And Vulcanization Mechanisms For Step 1 Download Scientific Diagram
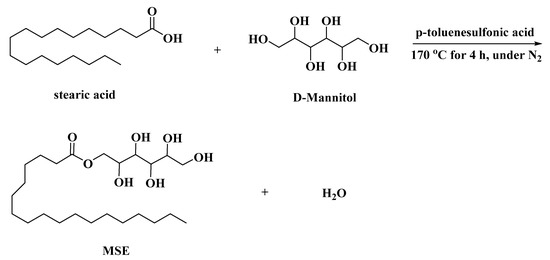
Polymers Free Full Text Design And Synthesis Of A New Mannitol Stearate Ester Based Aluminum Alkoxide As A Novel Tri Functional Additive For Poly Vinyl Chloride And Its Synergistic Effect With Zinc Stearate

Zinc Oxide Surface A Versatile Nanoplatform For Solvent Free Synthesis Of Diverse Isatin Derivatives Sciencedirect

Influence Of Two Different Alcohols In The Esterification Of Fatty Acids Over Layered Zinc Stearate Palmitate Sciencedirect

Scielo Brasil Influence Of Zno On The Properties Of Elastomeric Compositions And Their Leached Extract Influence Of Zno On The Properties Of Elastomeric Compositions And Their Leached Extract
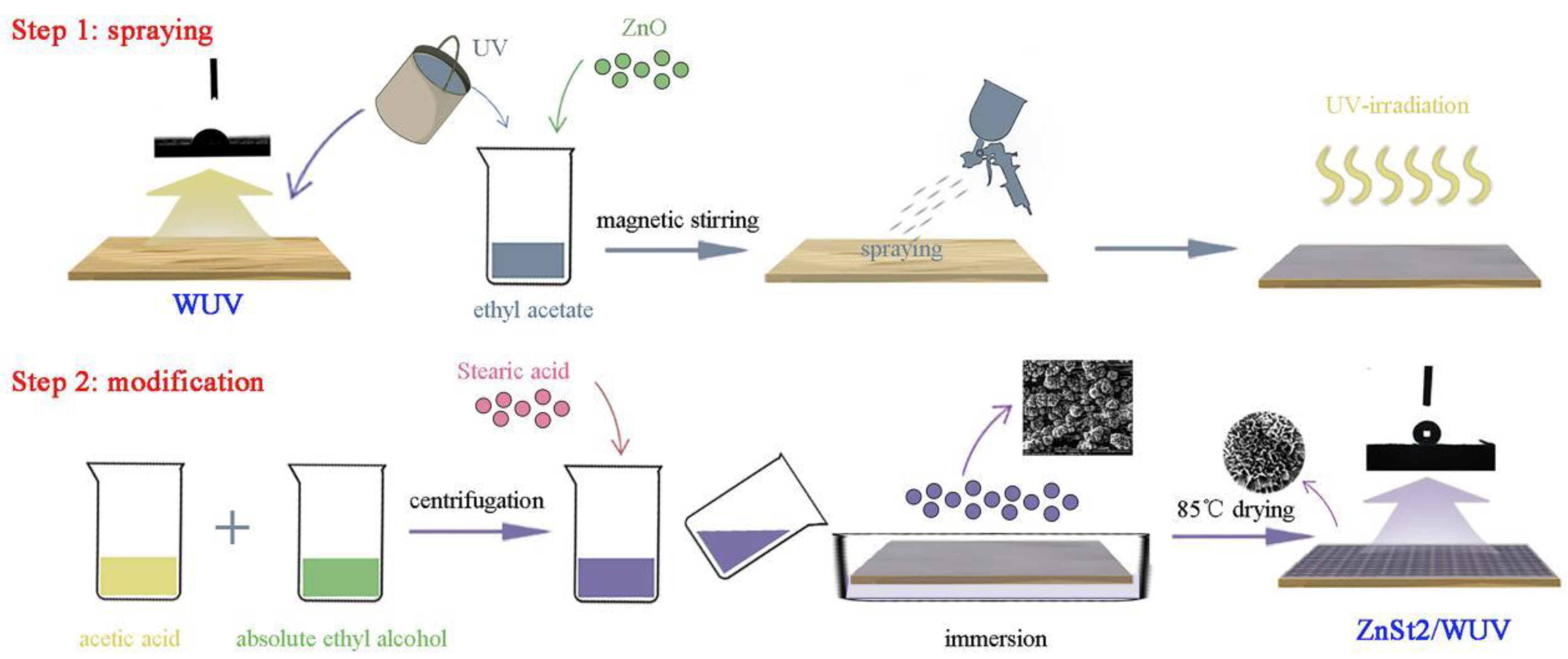
Polymers Free Full Text Preparation And Characterization Of Waterborne Uv Lacquer Product Modified By Zinc Oxide With Flower Shape Html

Tga Dsc Curves Of Zinc Stearate Reveal A Relative Thermal Equilibrium Download Scientific Diagram

Zinc Stearate Formation And Vulcanization Mechanisms For Step 1 Download Scientific Diagram

Zinc Stearate Technical Grade 557 05 1

Catalysts Free Full Text Zinc Based Curing Activators New Trends For Reducing Zinc Content In Rubber Vulcanization Process Html
Comments
Post a Comment